Description







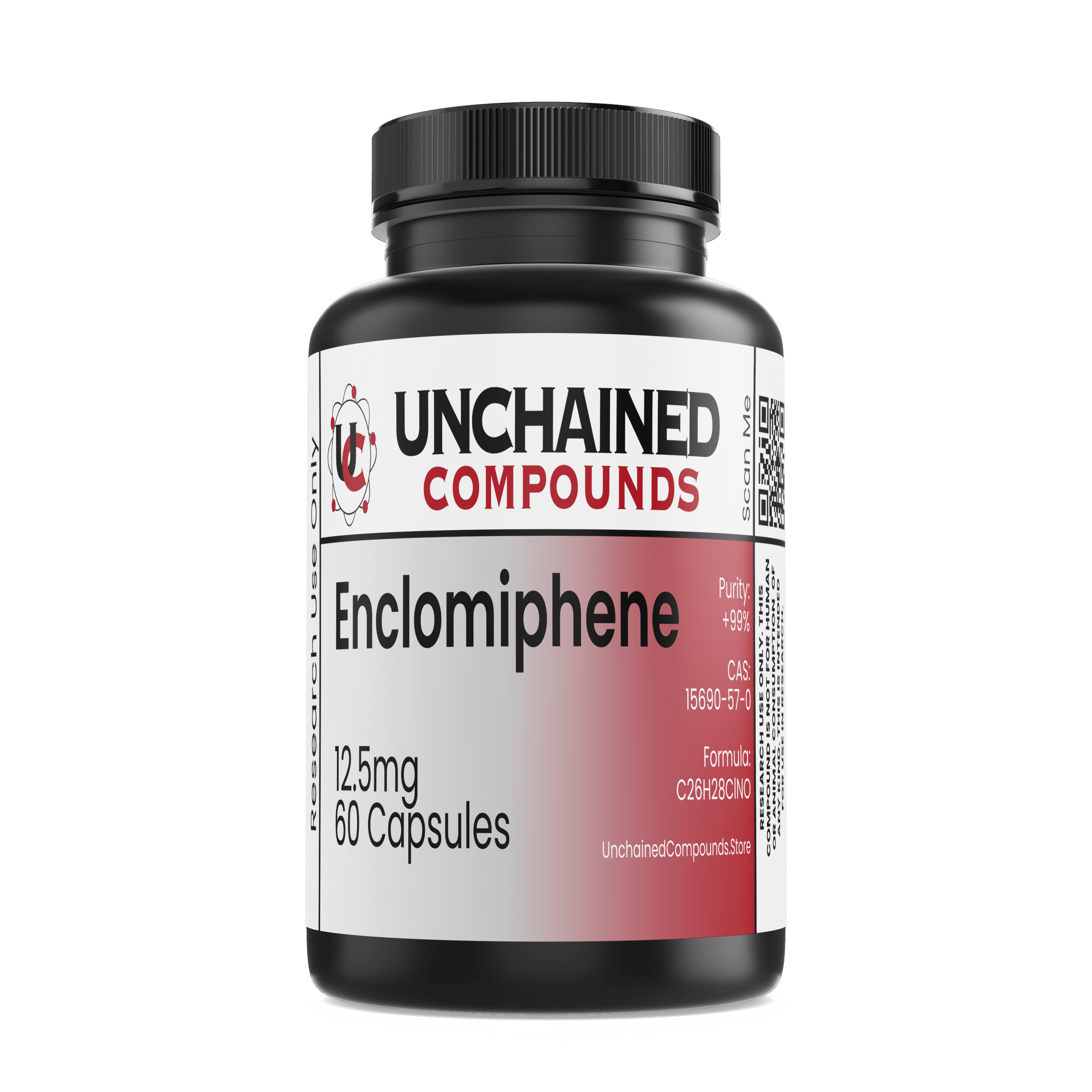
Enclomiphene is a selective estrogen receptor antagonist that increases gonadotropin secretion and hence gonadal production of testosterone. It is also known as Enclomifene, (E)-Clomifene, RMI-16289, Enclomid, and Enclomifene citrate.
Specifications
- Chemical Formula: C26H28C1N0
- Molecular Mass: 4405.974 g/mol
- Synonyms: Enclomifene, (E)-Clomifene, RMI-16289, Enclomifene citrate
- CAS Number: 15690-57-0
- PubChem: 1548953
- Total Amount of the Active Ingredient: 12.5mg/cap & 60 Capsules
- Shelf Life: 36 months
Minimize open air exposure, store in a cool dry place
Research Studies
Enclomiphene, an estrogen receptor antagonist for the treatment of testosterone deficiency in men
Abstract
Enclomiphene (Androxal), in development by Repros Therapeutics Inc, is a non-steroidal estrogen receptor antagonist that promotes gonadotropin-dependent testosterone secretion by the testes. Enclomiphene constitutes the trans-stereoisomer of clomiphene citrate, a drug that has been widely prescribed for several decades for the treatment of female ovulatory dysfunction. Because of the antagonistic effects of enclomiphene, the drug has the potential to increase serum testosterone levels in men with secondary hypogonadism by restoring physiological endogenous testosterone secretion while maintaining testicular volume and, potentially, spermatogenesis. In clinical trials conducted to date, enclomiphene demonstrated significant efficacy in the physiological restoration of testosterone levels in males with secondary hypogonadism. The compound also exhibited an unanticipated favorable effect on fasting plasma glucose; this result has been accompanied by rapidly accumulating evidence from other researchers for a bidirectional relationship between low serum testosterone and obesity/metabolic syndrome (syndrome X) in men. Short-term clinical safety data for enclomiphene have been satisfactory and equivalent to safety data for testosterone gels and placebo. Enclomiphene demonstrates promise in the management of secondary hypogonadism associated with obesity, metabolic syndrome and, possibly, infertility, and should undergo placebo-controlled, randomized clinical trials for these indications.
Enclomiphene citrate: A treatment that maintains fertility in men with secondary hypogonadism
Abstract
Hypogonadism is an important issue among the male population. Treatments such as exogenous testosterone have become very popular. One of the adverse effects of testosterone is its suppression of fertility. This has lead to the use of alternative therapies such as selective estrogen receptor modulators (SERMs) that aim to correct hypogonadism without reducing fertility. Areas covered: The SERM, clomiphene citrate, which is approved by the FDA for the treatment of ovarian dysfunction, has been shown to have beneficial effects on male hypogonadism. Clomiphene citrate exists as a mixture of both the cis-isomer (zuclomiphene) and the trans-isomer (enclomiphene). The literature has suggested that most of the beneficial effects of clomiphene are due to the trans-isomer enclomiphene. Zuclomiphene contributes little to the intended outcomes. The purpose of this drug profile is to examine the available literature on the trans-isomer enclomiphene. Expert opinion: Enclomiphene has been shown to increase testosterone levels while stimulating FSH and LH production. Initial studies demonstrated that enclomiphene maintains the androgenic benefit of clomiphene citrate without the undesirable effects attributable to zuclomiphene. This article reviews the difficulties associated with the FDA approval of a new molecular entity related to the treatment of hypogonadism.
Abstract
Objectives: To determine the effects of daily oral doses of enclomiphene citrate compared with topical testosterone gel treatment on serum total testosterone (TT), luteinising hormone (LH), follicle-stimulating hormone (FSH), and sperm counts in men with secondary hypogonadism.
Patients and methods: Two parallel randomised, double-blind, double-dummy, placebo-controlled, multicentre, phase III studies were undertaken to evaluate two doses of enclomiphene citrate vs testosterone gel (AndroGel(®) 1.62%) on TT, LH, FSH, and sperm counts in overweight men aged 18-60 years with secondary hypogonadism. Men were screened and enrolled in the trials (ZA-304 and ZA-305). All enrolled men had early morning serum TT levels in the low or low normal range (≤300 ng/dL; ≤10.4 nmol/L) and had low or normal LH (<9.4 IU/L) levels measured on two separate occasions 2-10 days apart. Serum samples were obtained over the course of the study to determine relevant hormone levels at baseline and after 16 weeks of treatment. Men provided semen samples twice to enroll at the beginning and twice at the end of the study.
Results: TT levels increased between baseline and after 16 weeks of treatment in all the treatment groups. FSH and LH levels increased in the enclomiphene citrate groups and decreased in the testosterone gel group at 16 weeks. Enclomiphene citrate maintained sperm concentration in the normal range over the treatment period, while there was a marked reduction in spermatogenesis in the testosterone gel group.
Conclusions: Enclomiphene citrate consistently increased serum TT, LH and FSH, restoring normal levels of serum TT. Enclomiphene citrate treatment maintained sperm concentrations in the normal range. The effects on TT were also seen with testosterone replacement via testosterone gel but sperm counts were not maintained.
Disclaimer
The information provided above is not intended to substitute medical advice, diagnosis, or treatment. Should you have any questions regarding a medical condition, seek the advice of your physician or a qualified healthcare provider. In no case should medical advice be disregarded or delayed because of what you have read or seen. We bear no responsibility or liability for your use of any of our research compounds and products. Please note that they are being sold for research purposes ONLY. We do NOT condone any personal use.
NOTE: In some cases wherein the assigned top colors are out of stock, a different top color will be used to ensure that your order will not be delayed. Should you need assistance identifying the peptide vial that you received, please send us an email at support@unchainedcompounds.store
ALL ARTICLES AND PRODUCT INFORMATION PROVIDED ON THIS WEBSITE ARE FOR INFORMATIONAL AND EDUCATIONAL PURPOSES ONLY.
The products offered on this website are intended for in-vitro studies only. In-vitro studies (Latin: “in glass”) are performed outside the body. These products are not medicines or drugs and have not been approved by the FDA to prevent, treat, or cure any medical condition, ailment, or disease. Bodily introduction of any kind into humans or animals is strictly forbidden by law.